



 Food Chemistry
Food Chemistry
Copyright © 2010 Elsevier Ltd All rights reserved. |
 Cited By in Scopus (0) Cited By in Scopus (0) |
 Permissions & Reprints Permissions & Reprints |

Lubin Zhanga, b, Guang Wangb, Jinmei Changa, Jianshuang Liub, Jianghua Caib, Xiuwen Raob, Lijuan Zhangb, Juanjuan Zhongb, Jianghui Xiea and Shijiang Zhub,  ,
, 
a Hainan Key Laboratory for Postharvest Physiology and Technology of Tropical Horticultural Products, South Subtropical Crops Research Institute, Chinese Academy of Tropical Agricultural Sciences, Zhanjiang 524091, Guangdong Province, China
b Guangdong Province Key Laboratory of Postharvest Physiology and Technology of Fruits and Vegetables, College of Horticulture, South China Agricultural University, Guangzhou 510642, Guangdong Province, China
Received 7 December 2009; revised 8 February 2010; accepted 30 March 2010. Available online 2 April 2010.Tsai Tai (Brassica chinensis) is produced mainly in China but also consumed overseas. The stems of Tsai Tai are subject to toughening due to lignification. Cinnamyl alcohol dehydrogenase (CAD) is a key enzyme involved in lignin biosynthesis. Three full-length CAD cDNAs were isolated, encoding proteins of 288, 323, and 323 amino acids, respectively. Sequence analysis showed that they share a highly conserved putative NAD/NADP(H)-binding site at the amino terminus. All the three BcCAD genes responded to 1-MCP and ethylene within 2 h. Ethylene up-regulated expression of BcCAD1-1 and BcCAD2, while 1-MCP down-regulated them. Ethylene increased yellow leaves and stem lignin, and 1-MCP decreased them. These results suggest ethylene is involved in lignin growth in Tsai Tai, and induced expression of BcCAD1-1 and BcCAD2 could contribute to lignification. They also suggest removal of ethylene and application of 1-MCP could extend shelf-life and knockout of certain CAD genes could produce lines that store well.
Keywords: Cinnamyl alcohol dehydrogenase; Ethylene; 1-MCP; Senescence; Lignification; Brassica chinensis
Primers used in the 5′- and 3′-RACE of the CAD cDNA.
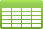
Note: FA, first amplification and SA, second amplification.
Sequences of primers for semi-quantitative RT-PCR.

 Corresponding author. Tel.: +86 20 38902512/13632355208; fax: +86 20 85288280.
Corresponding author. Tel.: +86 20 38902512/13632355208; fax: +86 20 85288280.
|
Food Chemistry Volume 123, Issue 1, 1 November 2010, Pages 32-40 |



 Food Chemistry
Food Chemistry
Copyright © 2010 Elsevier Ltd All rights reserved. |
 Cited By in Scopus (0) Cited By in Scopus (0) |
 Permissions & Reprints Permissions & Reprints |

Lubin Zhanga, b, Guang Wangb, Jinmei Changa, Jianshuang Liub, Jianghua Caib, Xiuwen Raob, Lijuan Zhangb, Juanjuan Zhongb, Jianghui Xiea and Shijiang Zhub,  ,
, 
a Hainan Key Laboratory for Postharvest Physiology and Technology of Tropical Horticultural Products, South Subtropical Crops Research Institute, Chinese Academy of Tropical Agricultural Sciences, Zhanjiang 524091, Guangdong Province, China
b Guangdong Province Key Laboratory of Postharvest Physiology and Technology of Fruits and Vegetables, College of Horticulture, South China Agricultural University, Guangzhou 510642, Guangdong Province, China
Received 7 December 2009; revised 8 February 2010; accepted 30 March 2010. Available online 2 April 2010.Tsai Tai (Brassica chinensis) is produced mainly in China but also consumed overseas. The stems of Tsai Tai are subject to toughening due to lignification. Cinnamyl alcohol dehydrogenase (CAD) is a key enzyme involved in lignin biosynthesis. Three full-length CAD cDNAs were isolated, encoding proteins of 288, 323, and 323 amino acids, respectively. Sequence analysis showed that they share a highly conserved putative NAD/NADP(H)-binding site at the amino terminus. All the three BcCAD genes responded to 1-MCP and ethylene within 2 h. Ethylene up-regulated expression of BcCAD1-1 and BcCAD2, while 1-MCP down-regulated them. Ethylene increased yellow leaves and stem lignin, and 1-MCP decreased them. These results suggest ethylene is involved in lignin growth in Tsai Tai, and induced expression of BcCAD1-1 and BcCAD2 could contribute to lignification. They also suggest removal of ethylene and application of 1-MCP could extend shelf-life and knockout of certain CAD genes could produce lines that store well.
Keywords: Cinnamyl alcohol dehydrogenase; Ethylene; 1-MCP; Senescence; Lignification; Brassica chinensis
Primers used in the 5′- and 3′-RACE of the CAD cDNA.

Note: FA, first amplification and SA, second amplification.
Sequences of primers for semi-quantitative RT-PCR.

 Corresponding author. Tel.: +86 20 38902512/13632355208; fax: +86 20 85288280.
Corresponding author. Tel.: +86 20 38902512/13632355208; fax: +86 20 85288280.
|
Food Chemistry Volume 123, Issue 1, 1 November 2010, Pages 32-40 |